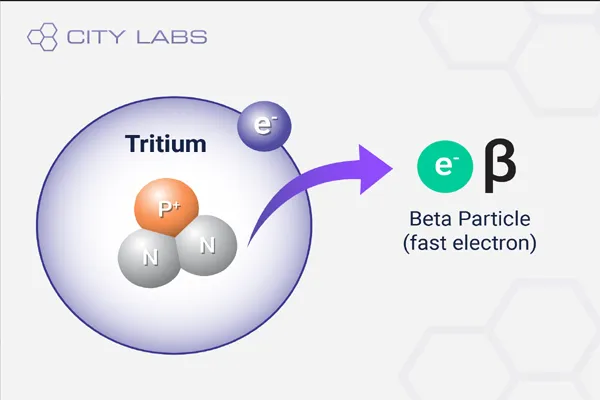
Identifying beta rays (β) and gamma rays (γ) involves distinguishing their properties, as they are both forms of ionizing radiation emitted during radioactive decay, but they differ significantly in composition and behavior. Beta rays are high-energy electrons (β⁻) or positrons (β⁺), while gamma rays are high-energy photons, a form of electromagnetic radiation. Here are some effective methods to identify and differentiate them:
1. Penetration Power
- Beta Rays: Have moderate penetration power. They can pass through a few millimeters of skin or paper but are stopped by a thin sheet of aluminum (e.g., 1-2 mm thick) or a few meters of air.
- Gamma Rays: Have very high penetration power. They can pass through thick materials like wood or human tissue and require dense shielding, such as several centimeters of lead or concrete, to be stopped.
- Method: Place different absorbers (e.g., paper, aluminum, lead) between the radiation source and a detector. If the radiation is stopped by aluminum but not paper, it’s likely beta. If it penetrates aluminum and requires lead to stop, it’s gamma.
2. Interaction with Magnetic Fields
- Beta Rays: Being charged particles (electrons or positrons), beta rays are deflected in a magnetic field. Electrons curve in one direction, positrons in the opposite, following the Lorentz force.
- Gamma Rays: As neutral photons, gamma rays are unaffected by magnetic fields and travel in a straight line.
- Method: Use a magnetic field (e.g., with a magnet or electromagnet) and observe the radiation path with a detector like a Geiger counter or cloud chamber. Deflection indicates beta rays; no deflection suggests gamma rays.
3. Ionization Capability
- Beta Rays: Cause moderate ionization in matter due to their charge, interacting with electrons in atoms as they pass through a medium like air or gas.
- Gamma Rays: Cause less direct ionization because they are uncharged. They primarily lose energy via the photoelectric effect, Compton scattering, or pair production, depending on their energy.
- Method: Use a detector like an ionization chamber or cloud chamber. Higher ionization tracks (thicker, shorter trails) suggest beta rays, while sparse, indirect ionization (faint or zigzag tracks) points to gamma rays.
4. Energy Spectrum
- Beta Rays: Exhibit a continuous energy spectrum, with energies ranging from near zero up to a maximum value specific to the isotope (e.g., 0.5–3 MeV typically).
- Gamma Rays: Have a discrete energy spectrum, with specific energy peaks corresponding to the nuclear transitions of the emitting isotope (e.g., 0.662 MeV for Cesium-137).
- Method: Use a scintillation detector or gamma spectrometer (e.g., NaI or HPGe detector) to measure the energy distribution. A broad, continuous spectrum indicates beta rays; sharp peaks indicate gamma rays.
5. Detection with Specific Instruments
- Beta Rays: Best detected with thin-window Geiger-Müller tubes or plastic scintillators, which allow the charged particles to enter while filtering out heavier particles like alpha.
- Gamma Rays: Detected efficiently by thicker scintillators (e.g., sodium iodide) or semiconductor detectors designed for high-energy photons.
- Method: Compare detector responses. A Geiger counter with a thin window detects both, but shielding with aluminum blocks beta rays, leaving gamma rays detectable.