1. Zeroth Law of Thermodynamics:
- Concept: This law states that if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
- Implication: It defines temperature as a fundamental and measurable property of matter. If two objects are at the same temperature, no heat flows between them.
2. First Law of Thermodynamics (Law of Energy Conservation):
- Concept: Energy cannot be created or destroyed, only transferred or transformed.
- Equation: ΔU=Q−W
- Explanation: The change in the internal energy (ΔU) of a system is equal to the heat (Q) added to the system minus the work (W) done by the system. This means that the energy added to a system can either increase its internal energy or be used to do work, or both.
3. Second Law of Thermodynamics:
- Concept: The entropy (disorder) of an isolated system never decreases over time. It can stay constant or increase, leading to the principle that natural processes tend to move towards a state of maximum entropy.
- Implication: This law explains why certain processes are irreversible, such as why heat flows from a hotter object to a cooler one, but not the reverse.
- Entropy and Efficiency: It also implies that no heat engine can be 100% efficient because some energy will always be lost as waste heat.
4. Third Law of Thermodynamics:
- Concept: As the temperature of a system approaches absolute zero (0 Kelvin), the entropy of a perfect crystal approaches zero.
- Implication: It is impossible to reach absolute zero because it would require an infinite amount of energy to remove the last bit of heat from a system.
Key Concepts in Thermodynamics:
- System: The part of the universe we are studying (e.g., a gas in a cylinder).
- Surroundings: Everything outside the system.
- State Functions: Properties like temperature, pressure, and volume, which depend only on the current state of the system, not on how it got there.
- Processes: Changes the system undergoes, such as isothermal (constant temperature), adiabatic (no heat exchange), isobaric (constant pressure), and isochoric (constant volume).
Applications:
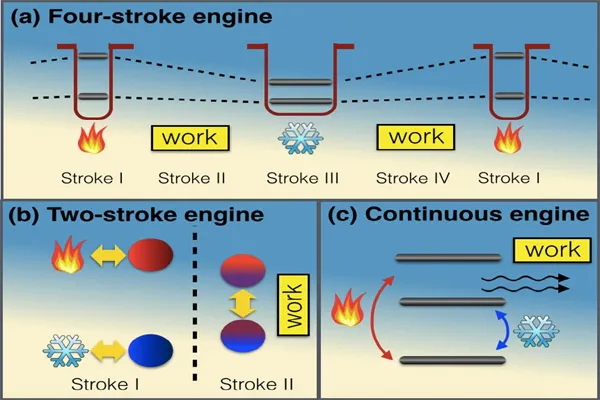
- Heat Engines: Devices that convert heat into work, like car engines or steam turbines.
- Refrigeration: Systems that use work to move heat from a colder to a hotter area, against the natural direction of heat flow.
- Chemical Reactions: Thermodynamics helps predict whether reactions are spontaneous, based on changes in enthalpy and entropy.
Thermodynamics is foundational in fields like chemistry, physics, engineering, and environmental science, explaining phenomena from the microscopic behavior of molecules to the workings of massive engines.